12 Month Study Results1
PROMISE II is a landmark multi-center, prospective pivotal study of the LimFlow System conducted at 20 U.S. centers in 105 No-option CLTI patients typically excluded from other clinical studies. All patients were confirmed as “No-Option” and facing imminent amputation by an independent review committee of vascular surgeons. Patients were enrolled between December 2020 to March 2022. This study was a success and met its primary endpoint.
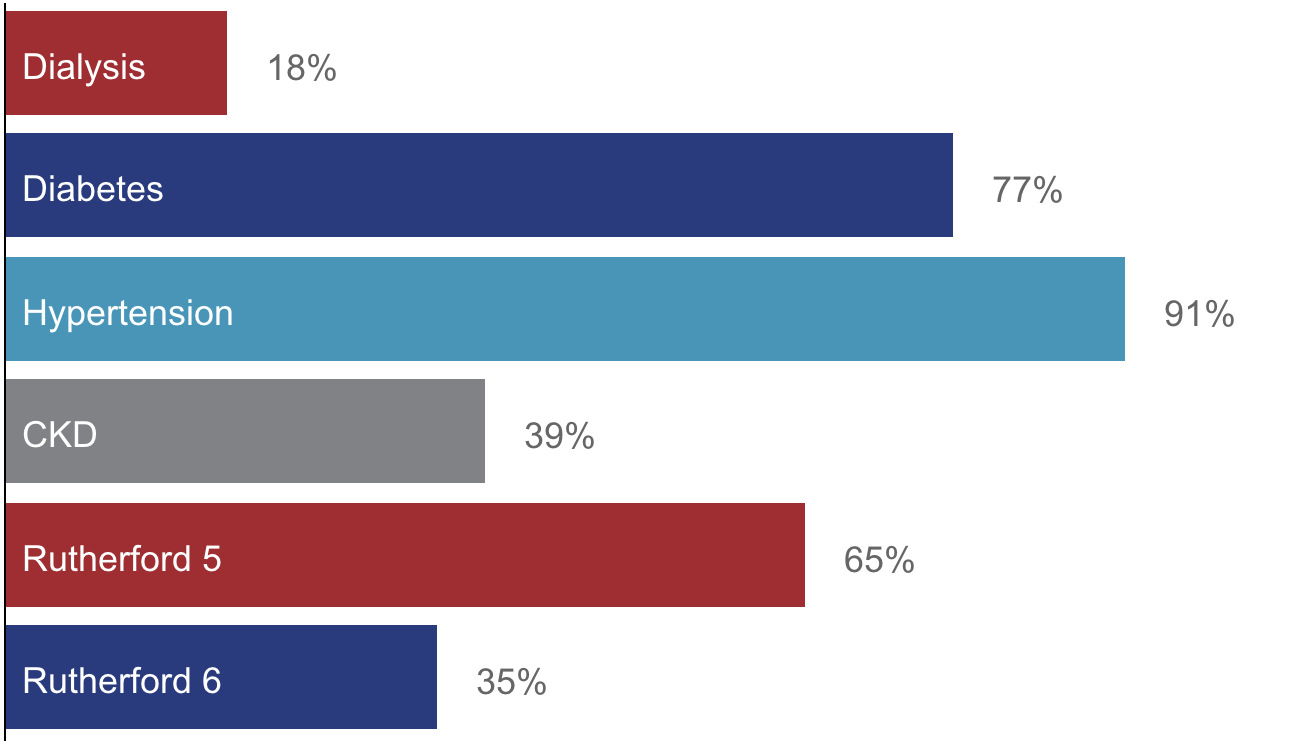
Patient Demographics
105 Patients
“Sickest population of CLTI patients ever enrolled in a pivotal trial.”
– Dr. Dan Clair, PROMISE II Co-PI
Limb Salvage at 12 Months
Functional Limb Preservation in No-Option Patients
Wounds Healed or Healing at 12 Months
Wound Healing in Patients With Non-Healing Chronic Wounds
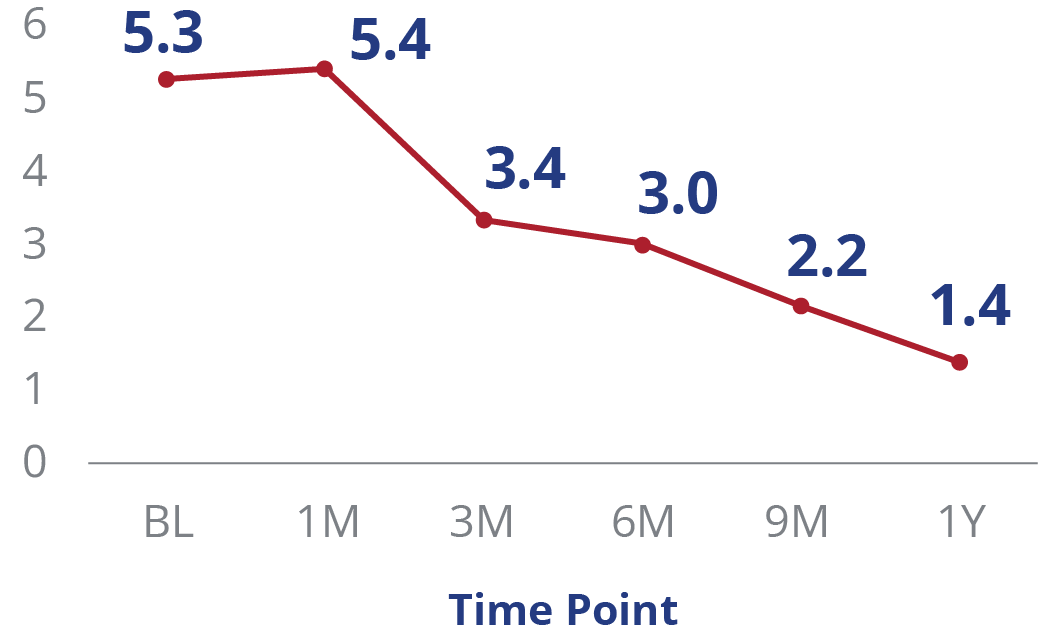
12M Pain Resolution and Rutherford Class Improvement
Pain Resolution (0-10 Scale)
Rutherford Classification Improvement
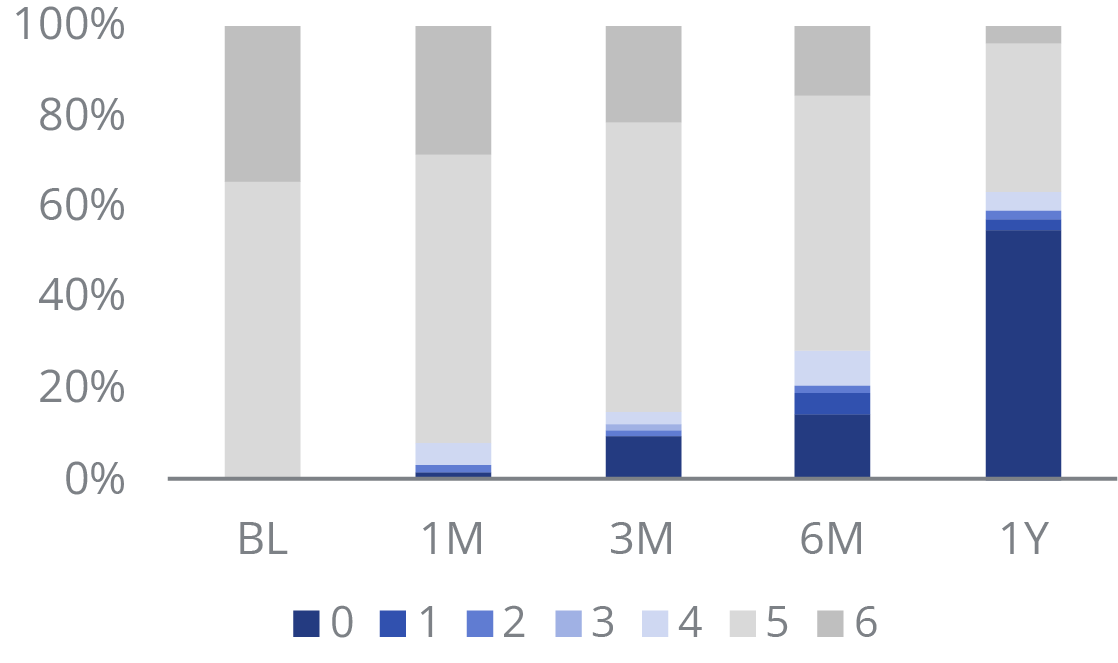
At 12 months, over half presented as Rutherford 0
Participating Sites:
| Institution | |
|---|---|
| Baylor College of Medicine | Sanger Heart & Vascular Institute |
| Boston Medical Center | Seton Heart Institute |
| Coastal Carolina Surgery | The Cardiac and Vascular Institute |
| Dartmouth – Hitchcock Medical Center | UH Cleveland Medical Center |
| Harbor-UCLA Medical Center | UnityPoint Health |
| Mass General Hospital | University of California San Francisco |
| Ochsner Health Center | University of Florida |
| Ponce Health Sciences University | Vanderbilt University Medical Center |
| Prisma Health Upstate and Midlands | Yale University |
| Saint Luke's Hospital of Kansas City | |
Thank you to all the investigators for your tremendous contribution to the PROMISE II pivotal trial.