Inari Medical is proud to offer comprehensive health economics and reimbursement resources to support transcatheter arterialization of the deep veins (TADV) using the LimFlow TADV System.* In addition to the resources provided here, our dedicated team of field-based Health Economics and Market Access (HEMA) Managers is available to assist with reimbursement education and questions.
Contact: Reimbursement@InariMedical.com
CMS Approves NTAP
CMS grants new technology add-on payment (NTAP) for the LimFlow System effective October 1, 2024.
Watch the video below to learn more:
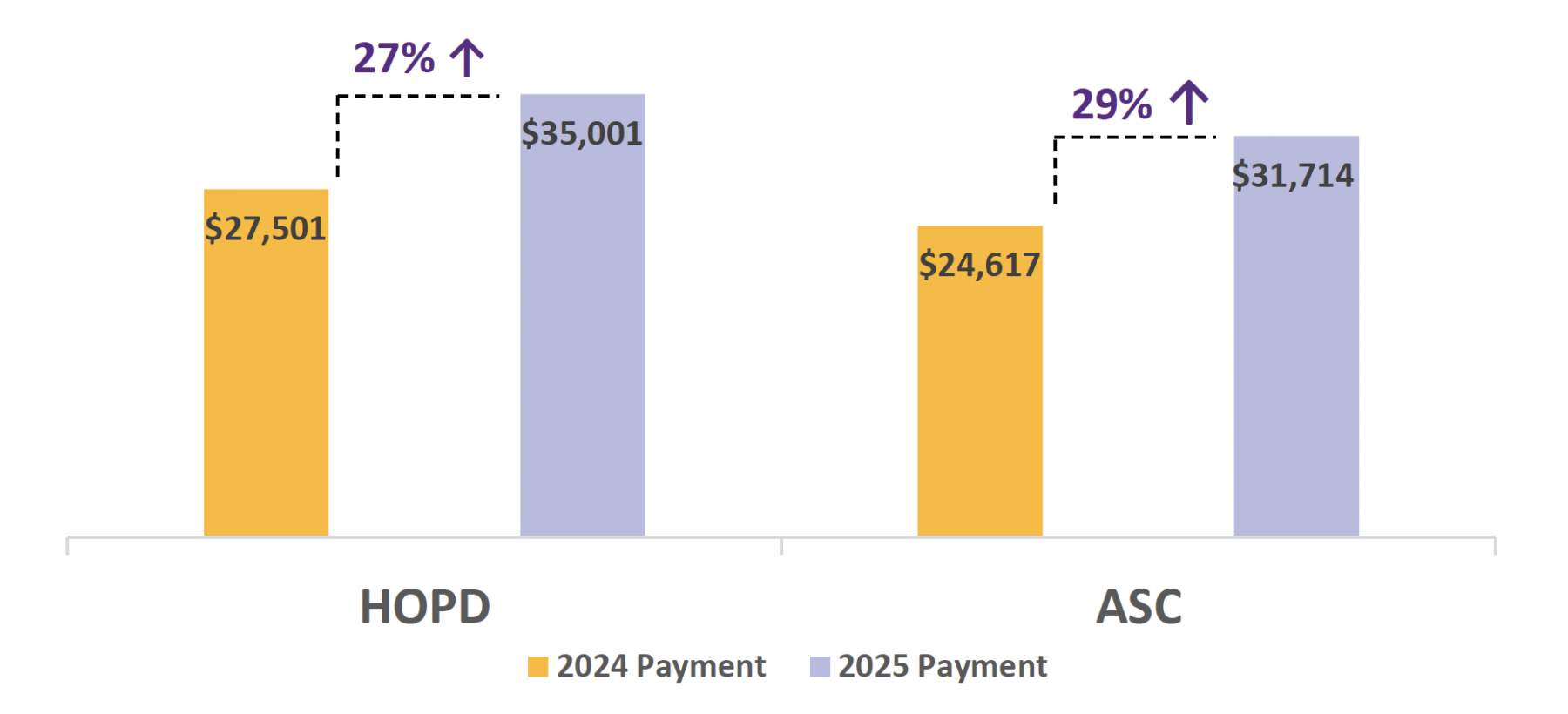
Medicare Increases Hospital Outpatient and ASC
Payment for LimFlow TADV
Effective January 1, 2025, Medicare will reassign the LimFlow TADV procedure (CPT 0620T) from New
Technology APC 1578 to New Technology APC 1579, which has an average payment amount of $35,001
in the hospital outpatient department (HOPD) and an average payment of $31,714 in the ambulatory
surgical center (ASC).
This positive reassignment represents a 27% increase in hospital outpatient patient and a 29% increase
in ASC payment for LimFlow TADV from 2024. Additionally, the facility payment for LimFlow TADV is
not subject to multiple procedure discounting. Medicare finalized these positive updates in the 2025
Medicare Hospital Outpatient Prospective Payment System Rule released on Friday, November 5, 2024.
YoY Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) National Average Medicare Payment
As a reminder, effective October 1, 2024, hospital inpatient LimFlow TADV cases are eligible for an
incremental payment of up to $16,250 from Medicare in addition to the applicable MS-DRG payment
for the case. This incremental reimbursement is called a “New Technology Add-on Payment” or NTAP.
Sources:
Medicare FFS Outpatient Hospital Rates effective January 1, 2024 through December 31, 2024.; Source: CY 2024 OPPS Final Rule; hospital impact file, Addendum A
Medicare FFS Outpatient Hospital Rates effective January 1, 2025 through December 31, 2025.; Source: CY 2025 OPPS Final Rule; hospital impact file, Addendum A
LimFlow NTAP finalized in FY 2025 IPPS Final Rule (CMS-1808-F).
Billing and Coding LimFlow TADV Procedures with Dr. Z
Gain valuable insights and expert guidance on billing and coding for these advanced procedures directly from Dr. Z, nationally recognized coding expert. During this on-demand webinar, Dr. Z will share best practices, tips, and strategies on billing and coding for LimFlow TADV procedures, ensuring appropriate reimbursement and and adherence to billing guidelines.
Attendees earn 1 CEU toward the American Academy of Professional Coders (AAPC) and is CIRCC certified. Also eligible for AHIMA CE credit
Resources to Download
Please complete the following form to access our available LimFlow reimbursement resources.
Disclaimer:
The coding and payment information contained in the above documents are publicly available from third party sources, and Inari Medical is providing it for general informational purposes only. The payments and/or costs are not all-inclusive and is neither intended to nor does it constitute legal, reimbursement, or business advice. The information is not a promise or guarantee by Inari Medical regarding actual costs or payment rates that providers will receive for any given service. Similarly, all CPT®, ICD-10 and HCPCS codes are supplied for informational purposes only and represent no statement or guarantee by Inari Medical that these codes are appropriate to specific circumstances or products or services provided to an individual patient or that the services will be covered. It is the health care provider’s responsibility to accurately report the patient diagnosis, the services provided, and the procedures performed, consistent with the payer’s guidelines. Likewise, site of service decisions (e.g., inpatient or outpatient) are based on medical necessity and should be determined by the physician in consultation with the patient and consistent with any payer guidelines or licensing provisions. If providers have questions about coverage, coding, or payment, providers should consult the specific payers. The Centers for Medicare and Medicaid Services (CMS) website is available at https://www.cms.gov/Medicare/Medicare.html.
Reimbursement is dynamic – payment rates change. New codes are added, and existing codes may be revised. Coverage policies also change. The information contained in this document is current as of the date of publication. CPT® Copyright 2025 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You are responsible for providing true, accurate and complete information concerning the applicable diagnosis and procedure codes and the patient’s medical record, and ensuring the medical necessity of the procedure. This letter is intended as an example for your consideration and may not include all the information necessary to support your prior authorization request. The requesting provider is entirely responsible for ensuring the accuracy, adequacy, and supportability of all information provided.
Important Safety Information
LimFlow System and LimFlow Stent Grafts Intended Use/Indications for Use: The LimFlow System and LimFlow Stent Grafts are indicated for patients who have chronic limb-threatening ischemia with no suitable endovascular or surgical revascularization options and are at risk of major amputation. Contraindications: Patients with deep venous thrombus in target vein; Patients with uncorrected bleeding disorders or patients who cannot receive anticoagulation or antiplatelet aggregation therapy. Warnings and Precautions: Use in patients with concomitant hepatic insufficiency has not been evaluated; Use in patients with poor cardiac output, e.g., NYHA Class IV, has not been evaluated; Use in pregnant and breastfeeding women has not been evaluated; Implanting the device in the distal half of the calcaneus may result in stent fracture. Adverse Events: Acute renal impairment requiring dialysis; Cardiac arrest, Death, Embolization, Graft rupture, trans-graft leak, site leak; Hematoma; Insufficient blood flow to foot; Ischemia; Myocardial infarction; Occlusion; Pain; Peripheral edema; Procedural bleeding; Restenosis of stented segment; Sepsis / Infection; Stent damage, implant migration; Stent graft fracture; Stent graft misplacement, deformation, or migration; The need for surgical or endovascular interventions to rectify an access site problem; Thrombosis; Vessel dissection, perforation, injury; Vessel spasm. Review complete Instructions for Use, Indications for Use, Warnings, Precautions, Possible Adverse Effects and Contraindications prior to use of the product. LimFlow ARC Intended Use/Indications for Use: The LimFlow ARC is intended to facilitate placement and positioning of guidewires and catheters within the peripheral vasculature. The LimFlow ARC is not intended for use in the coronary or cerebral vasculature. LimFlow V-Ceiver Intended Use/Indications for Use: The LimFlow V-Ceiver is intended for use in the cardiovascular system to manipulate and retrieve guidewires specified in the IFU.
LimFlow Vector Intended Use/Indications for Use: The LimFlow Vector is intended for the treatment of vascular disorders and more particularly for excising or disrupting venous valves.
Important Information: Review complete Instructions for Use, Indications for Use, Warnings, Precautions, Possible Adverse Effects and Contraindications prior to use of the product.
*LimFlow® TADV System includes the LimFlow System or LimFlow Stent Grafts, and LimFlow ARC, LimFlow Vector, and LimFlow V-Ceiver. Please refer to the Important Safety Information for these products for more details.
For all non-Inari products, please review manufacturer complete Instructions for Use, Indications for Use, Warnings, Precautions, Possible Adverse Effects and Contraindications.
Caution: Federal (USA) law restricts these devices to sale by or on the order of a licensed healthcare practitioner.
Copyright © 2025 LimFlow, Inc. All rights reserved.
LIMFLOW is a registered trademarks of LimFlow SA in the U.S. and other countries.
LimFlow is a proud member of the Inari Medical Family.
